Your Explain how colors in the flame test are produced images are ready. Explain how colors in the flame test are produced are a topic that is being searched for and liked by netizens now. You can Get the Explain how colors in the flame test are produced files here. Download all royalty-free images.
If you’re looking for explain how colors in the flame test are produced pictures information linked to the explain how colors in the flame test are produced interest, you have pay a visit to the right site. Our website frequently gives you suggestions for refferencing the maximum quality video and picture content, please kindly hunt and find more enlightening video content and graphics that fit your interests.
Explain How Colors In The Flame Test Are Produced. Not all metal ions give flame colours. The wavelength colour of the light depends on the difference in the two energy levels. Therefore the color of the light can be. This is the.
 Flame Test Red Green Blue Violet Activity Teachengineering From teachengineering.org
Flame Test Red Green Blue Violet Activity Teachengineering From teachengineering.org
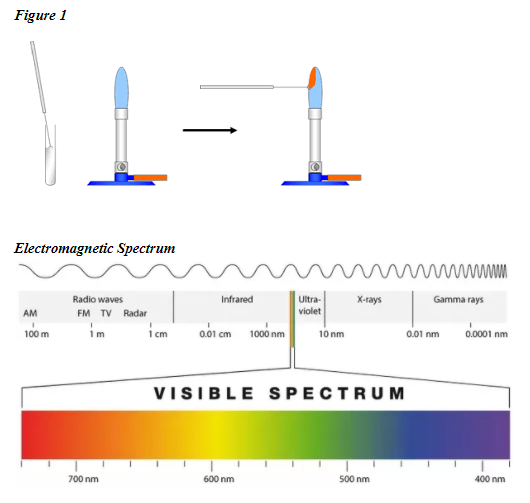
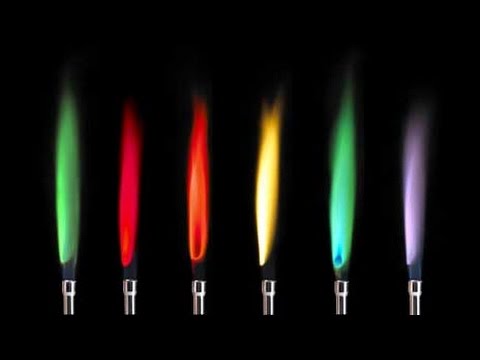
These element-specific colors are catalogued in an emission spectrum. Also asked why do different chemicals emit different colors of light in the flame test. The colors are produced when an electron jumps to a higher level and then jump back down. The orange yellow and red colors in a flame do not relate only to color temperature. The Bohr model says that electrons exist only at certain allowed energy levels. When the energy released in the visible light spectrum a certain color can be seen.
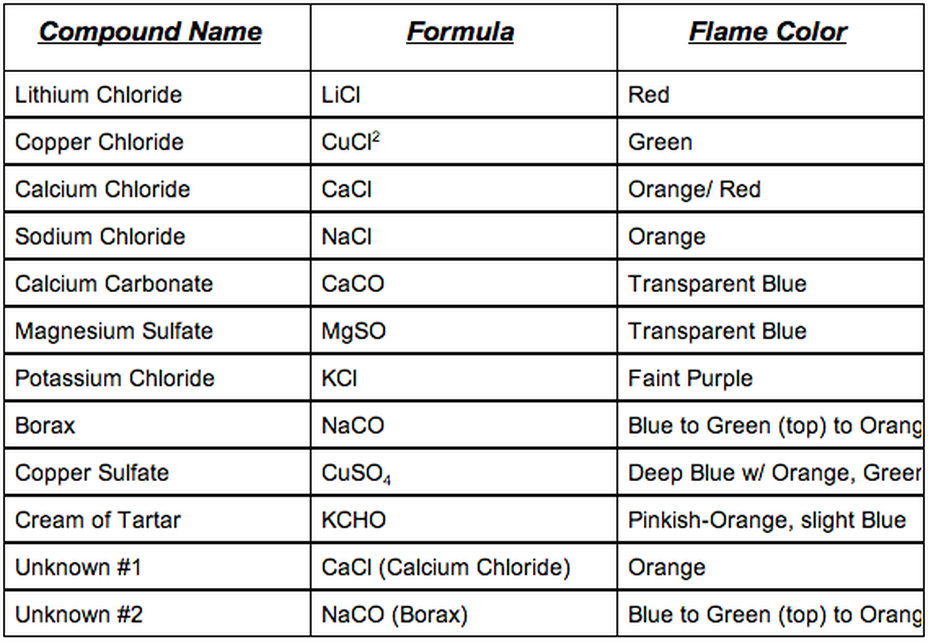
Green flame a trail of dark brown light green-ish streaks.
In chemistry terms the fact some metals burn with a characteristic flame colour is important since it allows us to introduce the concept of spectroscopy. When the energy released in the visible light spectrum a certain color can be seen. The color of this light can be used as a means of identifying the elements involved. The colors are produced when an electron jumps to a higher level and then jump back down. Green flame a trail of dark brown light green-ish streaks. Ions produce different flame colours when they are heated strongly.
 Source: slideplayer.com
Source: slideplayer.com
When you heat an atom some of its electrons are excited to higher energy levels. The pairs with similar colours were Ba2 and Cu2 and Sr2 and Li. Electrons quickly return to ground state emitting a discrete amount of energy as a photon of light. It is possible to create a variety of coloured flames by burning a small amount of different metal salts in a fire. The purpose of the flame test lab is to observe the characteristics colors produced by certain metallic Ions when vaporized in a flame.
 Source: sites.google.com
Source: sites.google.com
Identify unknown metallic Ions by means of its flame test. The atom absorbs the energy and electrons jump to higher discrete energy levels. Flame tests are used to identify the presence of a relatively small number of metal ions in a compound. Green flame a trail of dark brown light green-ish streaks. The colors are produced when an electron jumps to a higher level and then jump back down.

Explain what is responsible for the colors during a flame test. For Group 1 compounds flame tests are usually by far the easiest way of identifying which metal you have got. Gas excitations also play a major role in flame color. The colors observed. The color of the flame produced during a flame test is a characteristic of particular metallic elements.
 Source: mrpalermo.com
Source: mrpalermo.com
As the electrons return to their normal levels the energy that was absorbed is emitted in the form of electromagnetic energy. Explain how the colors in the flame tests are produced in terms of electrons and energy states. Thats because cream of tartar is a potassium salt. Also asked why do different chemicals emit different colors of light in the flame test. To carry out the flame tests a small amount of the compound being tested will be held in a flame and the colour given off observed.
 Source: teachengineering.org
Source: teachengineering.org
Not all metal ions give flame colours. The red portion is around 1070 K 800 C. The color inside the flame becomes yellow orange and finally red. Orange flame left a thin trail to the middle some salt in the middle. Many metallic ions exhibit characteristic colors when heated.
 Source: emr.ac.uk
Source: emr.ac.uk
Different elements produced distinct colors in the flame test due to their electrons falling from an excited state back to their ground state. When a compound containing one of the metallic elements is heated its atoms emit light of a specific color. The atom absorbs the energy and electrons jump to higher discrete energy levels. Sometimes barium produces a yellow flame without noticeable green. Therefore the color of the light can be.

Become a member and unlock. Sometimes barium produces a yellow flame without noticeable green. The color inside the flame becomes yellow orange and finally red. Ions produce different flame colours when they are heated strongly. When the energy released in the visible light spectrum a certain color can be seen.
 Source: study.com
Source: study.com
Sometimes barium produces a yellow flame without noticeable green. Some of this energy may be in the form of visible light. Does the presence of a solution affect the color produced by each element in a flame test. The wavelength colour of the light depends on the difference in the two energy levels. Blue flame until it reaches the middle becomes red-ish orange.

What pairs of ions produce similar colours in the flame test. For Group 1 compounds flame tests are usually by far the easiest way of identifying which metal you have got. Also asked why do different chemicals emit different colors of light in the flame test. When you heat an atom some of its electrons are excited to higher energy levels. As the electrons return to their normal levels the energy that was absorbed is emitted in the form of electromagnetic energy.
 Source: researchgate.net
Source: researchgate.net
For Group 1 compounds flame tests are usually by far the easiest way of identifying which metal you have got. The identity of the anion and the concentration of the chemical matter. When a compound containing one of the metallic elements is heated its atoms emit light of a specific color. The orange yellow and red colors in a flame do not relate only to color temperature. When an electron drops from one level to a lower energy level it emits a quantum of energy.
 Source: studylib.net
Source: studylib.net
Blue flame until it reaches the middle becomes red-ish orange. This is the basis of fireworks. Identify unknown metallic Ions by means of its flame test. Different elements produced distinct colors in the flame test due to their electrons falling from an excited state back to their ground state. Does the presence of a solution affect the color produced by each element in a flame test.
 Source: studylib.net
Source: studylib.net
Also asked why do different chemicals emit different colors of light in the flame test. To carry out the flame tests a small amount of the compound being tested will be held in a flame and the colour given off observed. The orange yellow and red colors in a flame do not relate only to color temperature. The cream of tartar yielded a purple-colored flame. Some of this energy may be in the form of visible light.
 Source: cshakira1.blogspot.com
Source: cshakira1.blogspot.com
Ions produce different flame colours when they are heated strongly. For this lab we got a whole bunch of compounds and burned them to observe the flame and frequency based off the color of the flame. Identify unknown metallic Ions by means of its flame test. Started off blue became red-ish when it reached the middle white trail. What is the process that produces the colors seen in the flame tests.
 Source: hightechhigh-faithsdp.weebly.com
Source: hightechhigh-faithsdp.weebly.com
Started off blue became red-ish when it reached the middle white trail. It is possible to create a variety of coloured flames by burning a small amount of different metal salts in a fire. Started off blue became red-ish when it reached the middle white trail. Flame tests are used to identify the presence of a relatively small number of metal ions in a compound. The atom absorbs the energy and electrons jump to higher discrete energy levels.
 Source: chegg.com
Source: chegg.com
Become a member and unlock. Use your periodic table of elements to explain the reason for this. The atom absorbs the energy and electrons jump to higher discrete energy levels. The color of the light depends on the energy change that took place wavelength and frequency. What pairs of ions produce similar colours in the flame test.
 Source: youtube.com
Source: youtube.com
The colors are produced when an electron jumps to a higher level and then jump back down. Thats because cream of tartar is a potassium salt. Electrons quickly return to ground state emitting a discrete amount of energy as a photon of light. As the electrons return to their normal levels the energy that was absorbed is emitted in the form of electromagnetic energy. The identity of the anion and the concentration of the chemical matter.

The purpose of the flame test lab is to observe the characteristics colors produced by certain metallic Ions when vaporized in a flame. When the energy released in the visible light spectrum a certain color can be seen. In chemistry terms the fact some metals burn with a characteristic flame colour is important since it allows us to introduce the concept of spectroscopy. Green flame a trail of dark brown light green-ish streaks. When you heat an atom some of its electrons are excited to higher energy levels.

Ba and Cu had light green-ish colours and Sr and Li had red colours. For Group 1 compounds flame tests are usually by far the easiest way of identifying which metal you have got. When the energy released in the visible light spectrum a certain color can be seen. This is the basis of fireworks. Many metallic ions exhibit characteristic colors when heated.
This site is an open community for users to share their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site serviceableness, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title explain how colors in the flame test are produced by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






